Top Chemistry Formulas
Here you will find the most common chemistry equations and formulas used in high school and fundamental university courses. These include, but are not limited to, temperature conversions, mole equations, pH, and ideal gas laws.
For more chemistry formulas visit Wikipedia.
Basic Equations
Atoms to Moles
A mole is an SI unit for measuring an amount of substance. The number of moles of anything can be calculated by taking the number of particles, (atoms, things, etc.) and dividing it by Avogadro’s number.
| # Moles = (#Particles) x |
|

Fahrenheit to Celsius
Most chemistry equations use temperature in degrees C or Kelvins. If given temperature in F, you can convert to C with the following equation.
| ℃ = (℉ - 32) x |
|
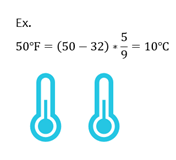
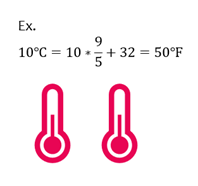
Celsius to Fahrenheit
If given temperature in C, you can convert to F with the following equation.
| ℉ = (℃ x |
|
) + 32 |
Celsius to Kelvins
If given temperature in C, you can convert to K with the following equation.
| K = ℃ + 273.15 |
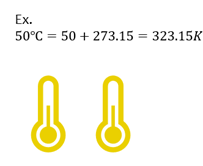
Solutions
Molarity
Molarity is a measure of solution concentration which is calculated as the moles of solute per liter of solution.
| moles solute |
| L solution |
Molality
Molality is a measure of solution concentration which is calculated as the moles of solute per kilogram of solvent.
| moles solute |
| kg solvent |
pH & pOH
pH = - log[H+]
pOH = - log log[OH-]
pH specifies the acidity or basicity of a solution, where [H+] means the concentration of hydrogen ions and [OH-] is the concentration of hydroxide ions.
Henderson-Hasselbalch Acid Dissociation
Relationship between pH and pKa
| pH = pKa + |
|
= pKa + |
|
Atomic Structure
DeBroglie
Particles can behave like waves. A wavelength can be given to a particle if the mass m and velocity v is known. h is Planck’s constant.
| λ = |
|
Planck Einstein Relation
Where h is Planck’s constant
| E = hf = |
|
||
| c = λf |
Gases
Boyle’s Law
Used when temperature is constant. P1 and V1 are the initial pressure and volume, and P2 and V2 are the new volume and pressure.
| P1V1 = P2V2 |
Charles’ Law
Used when pressure is constant. V1 and T1 are the initial volume and temperature, and T2 and V2 are the new temperature and pressure.
|
= |
|
Ideal Gas
Where R is the ideal gas constant
| PV = nRT |
Thermodynamics
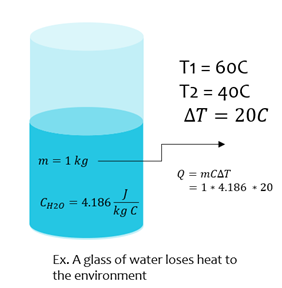
Heat Energy
Where m is mass of a substance (kg), c is the specific heat (J/kg∙K), and ∆T is change in temperature (Kelvins, K or Celsius, C)
| Q = mC ΔT |